Coley’s Fluids
Using the immune system to fight disease is not a new idea. As far back as the 18th century, Lady Wortley Montague, the wife of the British ambassador to Istanbul, noticed that the Turks were preventing smallpox by a technique similar to smallpox and brought the technique back to England. In fact, there is even evidence in traditional Chinese that the Chinese had the same idea for preventing smallpox much earlier.
But since there wasn’t much knowledge about cancer then, it was treated differently — as a disease to be cut out. That’s how a surgeon named William Coley became interested in immunotherapy at the end of the 19th century. Coley worked at a hospital with the less-than-inspiring name of the Hospital for the Ruptured and Crippled. His first patient died of metastatic sarcoma.
Young and still impressionable, Coley began doing research and found 47 examples of patients who experienced miraculous remissions that were supposedly caused by infections they had at the same time as the cancer. Somehow, these infections must have triggered something in their immune systems that “cured” their cancers.
The first connection he observed was one between erysipelas, a strep infection of the skin, and sarcomas. But he actually had a couple of patients die of septicemia on the way to his discoveries, because once he infected them with strep, he couldn’t cure them. He had to stumble on a combination of gram negative and gram positive bacteria before he could produce results without the potential “side effect” of death:
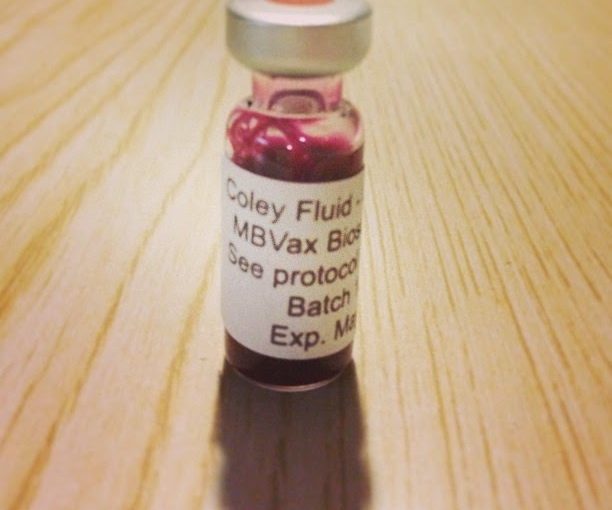
his fortuitous combination of Gram-positive and Gram-negative bacteria possessed a wide array of immunostimulatory properties that allowed Dr. Coley to achieve excellent long-term cure rates that in some instances remain unrivaled by medical science in the 81 years since his death (14–18). Despite impressive clinical results first published in 1893, Dr. Coley was viewed with suspicion by the medical establishment of the day; and while Paul Ehrlich would propose the cancer immunosurveillance hypothesis only 16 years later (19), contemporaries didn’t make a connection between “Coley fluid” and the nascent science of immunology. Therefore, in his own time, the lack of a suitable explanation for Coley’s results ultimately doomed his treatment regimen, and it is the century-long search for mechanism that has come to define William Coley’s legacy (20).
During his lifetime, Parke Davis signed on to produce his specific toxins, and they were deployed with mixed results, probably because no one yet understood enough about the immune system to understand how they worked.
Of course the medical establishment rejected “Coley’s toxins,” and by 1963 they could no longer be obtained in the US except for research studies. The establishment bet heavily on chemotherapy, which probably set the cause of immunotherapy back for a couple of decades.
However, a combination of events, such as the discovery of T-cells and interferon in the 1960s, focused the cancer research establishment back on immunotherapy and the first bone marrow transplants happened in the 70s.
The pace of immunotherapy research picked up dramatically during the early years of this century. “By the end of 2016, four different checkpoint inhibitor drugs blocking two different pathways (25–27, 51) had received FDA approval for the treatment of melanoma, renal cell carcinoma (RCC), lung cancer, lymphoma, and cancers of the bladder. numerous FDA approved regimes for immunotherapy oncology.”
More will come. This is an exciting field for patients, researchers, and providers.